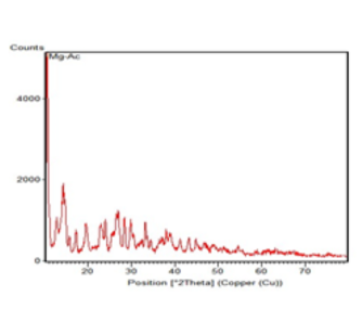
Magnesium acetate
specialized product area
product title
• chemical industry
• Biologically
laboratory sample
Current status of product operation
Magnesium acetate is in two anhydrous forms whit the chemical formula Mg(C2H3O2)2 and in hydrated form, (magnesium acetate tetrahydrate), with Mg(CH3COO)2 • 4H2O.
This product is in the form of wet white crystals and has a smell similar to acetic acid and is soluble in water. Magnesium acetate is commonly used as a source of magnesium in biological reactions.
It is also used as a source of magnesium or acetate ions in chemistry experiments. One of the most common uses of magnesium acetate is in a mixture called calcium magnesium acetate (CMA) as an environmentally friendly alternative to NaCl and CaCl2.
Magnesium acetate causes a structural change in Escherichia coli enzyme. Among other things, the use of this product when mixed with hydrogen peroxide acts as a bactericide.
Brief introduction of the product
Scope of application
chemistry and biotechnology
order product